Optimising Health and Athletic Performance

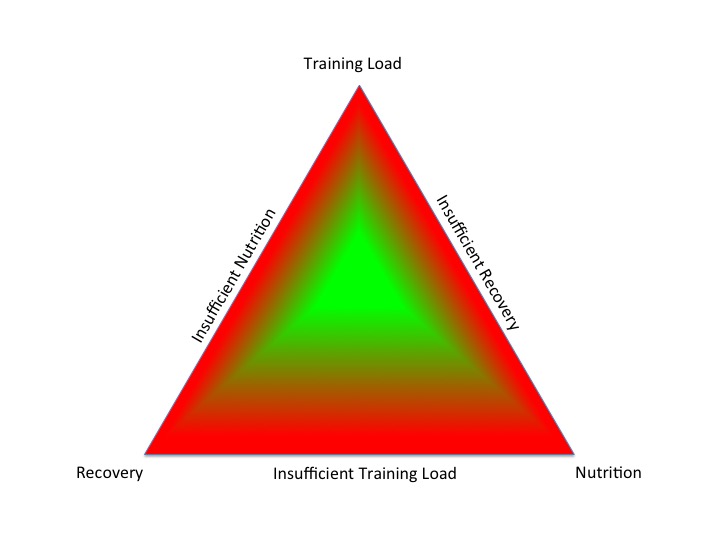
In order to improve sports performance, athletes periodise their training, nutrition and recovery within the context of a training season. For those not in exercise training, these controllable lifestyle factors correspond to exercise, diet and sleep, which require modification during the lifespan. In old money, this was called preventative medicine. Taking this a step further, rather than preventing disease, this proactive, personalised approach optimises health. Health should be a positive combination of physical, mental and social well being, not simply an absence of illness.
Failure to balance these lifestyle factors in an integrated fashion leads to negative outcomes. An athlete may experience maladaptation, rather than the desired adaptations to exercise training. For non-athletes an adverse combination of lifestyle factors can lead to suboptimal health and a predisposition to developing chronic disease.
What are the fundamental pathophysiological mechanisms involved in the aetiology of the clinical spectrum of suboptimal health, suboptimal sports performance and chronic disease?
Inflammation A degree of systemic inflammation and oxidative stress induced by exercise training is required to drive desired adaptations to support improved sport performance. However, prolonged, elevated levels of inflammation have adverse effects on health and underpin many chronic disease states. For example, inflammation is a contributing pathophysiological factor in the development of atherosclerosis and atherothrombosis in cardiovascular disease. What drives this over-response of the inflammatory process? Any combination of adverse lifestyle factors. Adipose tissue has an Endocrine function, releasing a subgroup of cytokines: adipokines which have peripheral and central signalling roles in energy homeostasis and inflammation. In a study of Belgian children, pro-inflammatory energy related biomarkers (high leptin and low adiponectin) were associated with decreased heart rate variability and hence in the long term increased risk of cardiovascular disease. For those with a pre-existing chronic inflammatory condition, response to treatment can be optimised with personalised lifestyle interventions.
Metabolism Non-integrated lifestyle factors can disrupt signalling pathways involved in glucose regulation, which can result in hyperinsulinaeamia and insulin resistance. This is the underlying pathological process in the aetiology of metabolic syndrome and metabolic inflexibility. Non-pharmacological interventions such as exercise and nutrition, synchronised with endogenous circadian rhythms, can improve these signalling pathways associated with insulin sensitivity at the mitochondrial level.
Intriguingly, evidence is emerging of the interaction between osteocalcin and insulin, in other words an Endocrine feedback mechanism linking bone and metabolic health. This is reflected clinically with increased fracture risk found amongst type 2 diabetics (T2DM) with longer duration and higher HbA1C.
Hormone imbalance The hypothalamus is the neuroendocrine gatekeeper of the Endocrine system. Internal feedback and external stimuli are integrated by the hypothalamus to produce an appropriate Endocrine response from the pituitary gland. The pathogenesis of metabolic syndrome involves disruption to the neuroendocrine control of energy homeostasis with resistance to hormones secreted from adipose tissue (leptin) and the stomach (ghrelin). Further evidence for the important network effects between the Endocrine and metabolic systems comes from polycystic ovarian syndrome (PCOS). Although women with this condition typically present to the Endocrine clinic, the underlying aetiology is metabolic dysfunction with insulin resistance disrupting the hypothamic-pituitary-ovarian axis. The same pathophysiology of disrupted metabolic signalling adversely impacting the hypothalamic-pituitary-gonadal axis also applies to males.
In athletes, the exact same signalling pathways and neuroendocrine systems are involved in the development of relative energy deficiency in sports (RED-S) where the underlying aetiology is imbalance in the periodisation of training load, nutrition and recovery.
Gastrointestinal tract In addition to malabsorption issues such as coeliac disease and non-gluten wheat sensitivity, there is emerging evidence that the composition and diversity of the gut microbiota plays a significant role in health. The microbiome of professional athletes differs from sedentary people, especially at a functional metabolic level. Conversely, an adverse gut microbiome is implicated in the pathogenesis of metabolic dysfunction such as obesity and T2DM, via modulation of enteroendocrine hormones regulating appetite centrally and insulin secretion peripherally.
Circadian disregulation As previously discussed, it is not just a question of what but WHEN you eat, sleep and exercise. If there is conflict in the timing of these lifestyle activities with internal biological clocks, then this can disrupt metabolic and endocrine signally. For example, in children curtailed sleep can impact glucose control and insulin sensitivity, predisposing to risk of developing T2DM. Eating too close to the onset of melatonin release in the evening can cause adverse body composition, irrespective of what you eat and activity levels. In those with pre-existing metabolic dysfunction, such as PCOS, timing of meals has an effect on insulin levels and hence reproductive Endocrine function. The immune system displays circadian rhythmicity which integrated with external cues (for example when we eat/exercise/sleep) optimises our immune response. For athletes competing in high intensity races, this may be more favourable in terms of Endocrine and metabolic status in the evening.
Psychology Psychological stress impacts the key pathophysiological mechanisms outlined above: metabolic signalling, inflammation and neuroendocrine regulation, which contribute to Endocrine and metabolic dysfunction. Fortunately stress is a modifiable lifestyle risk factor. In the case of functional hypothalamic amenorrhoea (where nutrition/exercise/sleep are balanced), psychological intervention can reverse this situation.
Conclusion Putting this all together, if the modifiable lifestyle factors of exercise, nutrition, sleep are optimised in terms of composition and timing, this improves metabolic and Endocrine signalling pathways, including neuroendocrine regulation. Preventative Medicine going beyond preventing disease; it optimises health.
BASEM annual conference 22/3/18: Health, Hormones and Human Performance
References
Athletic Fatigue: Part 2 Dr N. Keay
From population based norms to personalised medicine: Health, Fitness, Sports Performance Dr N. Keay, British Journal of Sports Medicine 2017
Endocrine system: balance and interplay in response to exercise training Dr N. Keay
Saturated fat does not clog the arteries: coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions British Journal of Sports Medicine 2017
Longitudinal Associations of Leptin and Adiponectin with Heart Rate Variability in Children Frontiers in Physiology 2017
Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals Sports Medicine 2017
Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus Nature Reviews Endocrinology
Insulin and osteocalcin: further evidence for a mutual cross-talk Endocrine 2017
HbA1c levels, diabetes duration linked to fracture risk Endocrine Today 2017
The cellular and molecular bases of leptin and ghrelin resistance in obesity Nature Reviews Endocrinology 2017
Metabolic and Endocrine System Networks Dr N. Keay
Adiponectin and resistin: potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species Reproduction 2017
Optimal Health: For All Athletes! Part 4 – Mechanisms Dr N. Keay, British Association of Sport and Exercise Medicine 2017
Ubiquitous Microbiome: impact on health, sport performance and disease Dr N. Keay
Interplay between gut microbiota, its metabolites and human metabolism: Dissecting cause from consequence Trends in Food Science & Technology 2016
Temporal considerations in Endocrine/Metabolic interactions Part 1 Dr N. Keay, British Journal of Sports Medicine 2017
Temporal considerations in Endocrine/Metabolic interactions Part 2 Dr N. Keay, British Journal of Sports Medicine 2017
Sleep Duration and Risk of Type 2 Diabetes Paediatrics 2017
Later circadian timing of food intake is associated with increased body fat Am J Clin Nutr. 2017
Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome Clin Sci (London)
Immunity around the clock Science
Type 2 diabetes mellitus and psychological stress — a modifiable risk factor Nature Reviews Endocrinology 2017